FASTEST GROWING OTC ALLERGY BRAND1
coh-inline-element button-primary animation-off coh-style-secondary-button remove-border-bottom COMPARE VS INS
PRN NASAL SYMPTOM RELIEF THAT STARTS WORKING IN
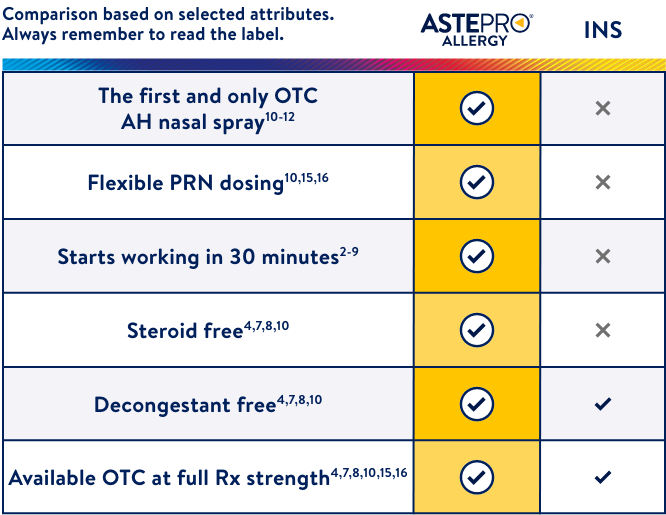
Astepro® Allergy is different than INS sprays
- Starts working in 30 minutes2,9,† while INS sprays take hours to start to provide relief and can take between 3 and 14 days to reach maximum efficacy levels3-8,‡
- Starts working in 30 minutes2,9,† while INS sprays take hours to start to provide relief and can take between 3 and 14 days to reach maximum efficacy levels3-8,‡
- Flexibility to dose PRN, once or twice a day, versus the leading allergy spray products that recommend daily dosing schedules that may not match patient usage behavior10,13,14,§
- Flexibility to dose PRN, once or twice a day, versus the leading allergy spray products that recommend daily dosing schedules that may not match patient usage behavior10,13,14,§
OTC ASTEPRO® OFFERS ADVANTAGES VERSUS RX AZELASTINE 0.10%
OTC ASTEPRO® OFFERS ADVANTAGES VERSUS RX AZELASTINE 0.10%
INS, intranasal steroid.
*Compared with steroid allergy sprays on day 1.
†On day 1.
‡INS onset based on various product information; time to maximum efficacy depends on the INS brand.
§In a survey of allergy patients that included nasal spray users (n=732), 42% of patients said they administer their INS “some days and not others.”13
||Up to twice daily.
REFERENCES:
1. Data on file. Bayer. Fastest growing. 2. Hsu SN, Sajjad F, Brigham E, et al. Onset of efficacy of azelastine hydrochloride 0.15% nasal spray for allergic rhinitis in an environmental exposure chamber. Ann Allergy Asthma Immunol. 2024;133(6):675-681. doi:10.1016/j.anai.2024.07.020 3. Flonase® website. Accessed March 18, 2024. https://www.flonase.com/faqs/ 4. Flonase®. Prescribing Information. GlaxoSmithKline; 2019. 5. Haleon Health Partner website. Accessed March 18, 2024. https://www.haleonhealthpartner.com/en-us/respiratory-health/brands/flonase-products/flonase/dosing-administration-flonase-allergy-relief/ 6. Nasacort® website. Accessed March 18, 2024. https://www.nasacort.com/en-us/allergy-nasal-spray 7. Nasacort® AQ. Prescribing information. Sanofi-aventis U.S. LLC; 2008. 8. Budesonide Nasal Spray. Prescribing information. Apotex Inc.; 2011. 9. Shah S, Berger W, Lumry W, La Force C, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray and azelastine 0.10% nasal spray in patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30(6):628-633. doi:10.2500/aap.2009.30.3296 10. NDA 213872. 11. FDA Approves a Nasal Antihistamine for Nonprescription Use. News release. PR Newswire; June 17, 2021. Accessed March 18, 2024. https://www.prnewswire.com/news-releases/fda-approves-a-nasal-antihistamine-for-nonprescription-use-301315152.html 12. Data on file. Bayer. IRI data. Only OTC AH. 13. Data on file. Bayer. Habits and practices. 14. Ortiz G, Knudtson M. Counseling patients on the use of intransal steroids in allergic rhinitis management. Consultant. 2017;57(6):328-334. 15. Flonase® Drug Facts Label. NDA205434. 16. Nasacort® Drug Facts Label. NDA020468. 17. Astepro®. Prescribing information. Meda Pharmaceuticals Inc; 2018. 18. US Department of Health and Human Services. Pediatric Postmarketing Pharmacovigilance and Drug Utilization Review. November 3, 2016. Accessed February 28, 2024. 19. Astelin. Prescribing information. Meda Pharmaceuticals Inc; 2014. 20. van Bavel J, Howland WC, Amar NJ, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray administered once daily in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30(5):512-518. doi:10.2500/aap.2009.30.3284