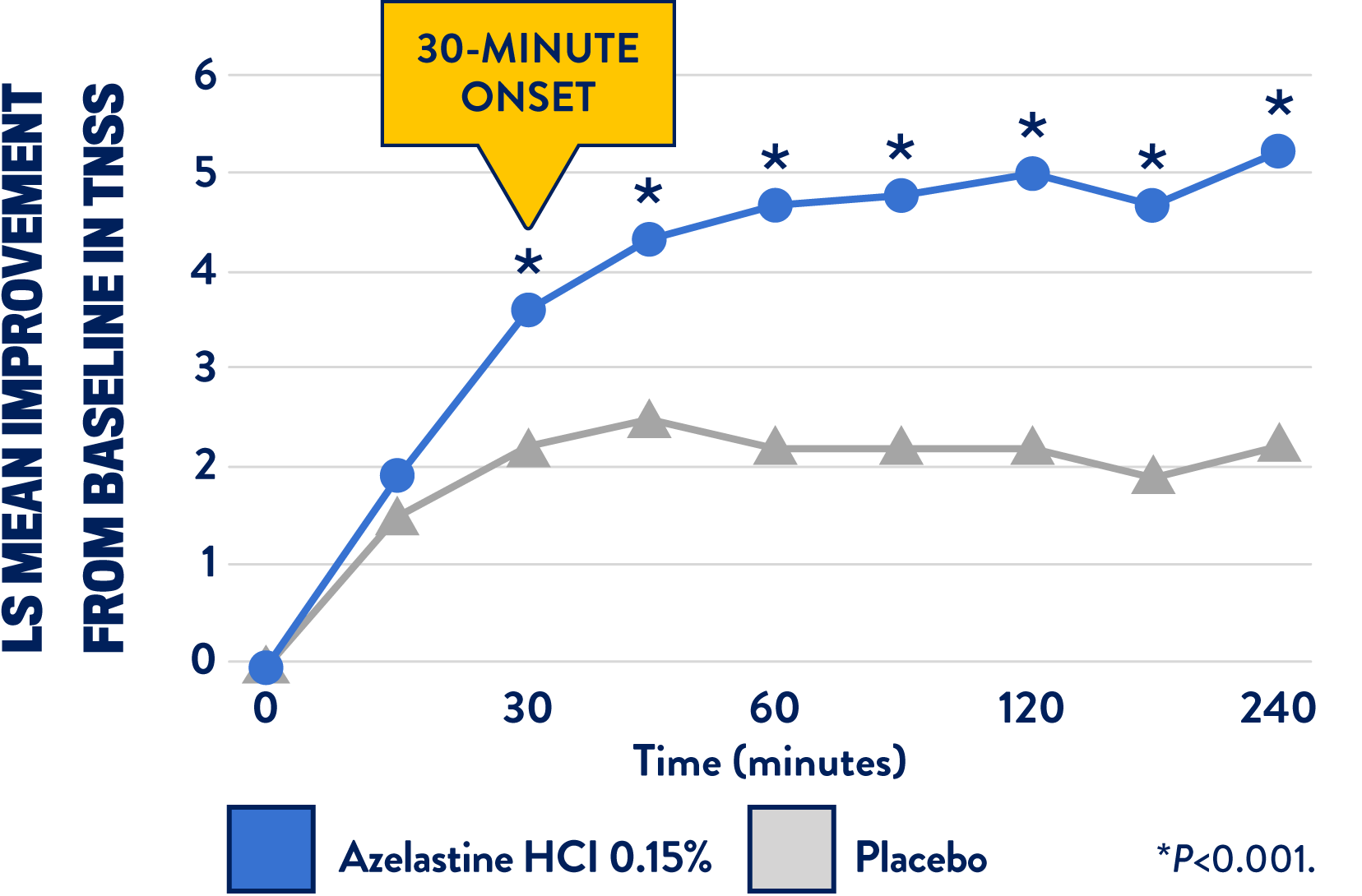
IN A RECENT STUDY, ASTEPRO® ALLERGY DEMONSTRATED A 30-MINUTE ONSET OF ACTION IN THE TESTED POPULATION1
Watch Dr. Nabeel Farooqui present highlights of the study in a clinical review
At 30 minutes, the results show a statistically significant and clinically meaningful difference between Astepro® and placebo1
Study details:
- The study was a phase 3, single-center, double-blinded, placebo-controlled crossover study evaluating the time to onset of efficacy of azelastine HCl 0.15% vs placebo in the relief of nasal symptoms1
- Subjects, healthy adults (18-65 years old) suffering from seasonal allergic rhinitis, were divided into 2 groups, receiving Astepro® or placebo1
Astepro® starts working in 30 minutes, while INS sprays can take up to 12 hours to provide relief on day 1. INS sprays can take 3 to 14 days to reach maximum efficacy levels.1-8
ASTEPRO SIGNIFICANTLY IMPROVED ALL 4 TNSS COMPONENTS2,9-11
Results of a 2-week, placebo-controlled, double-blind study comparing azelastine HCI 0.15% nasal spray, 2 sprays per nostril once daily, with placebo in patients with SAR (n=266 for patients receiving Astepro and n=266 for patients receiving placebo). The study was conducted in the United States during Texas Mountain Cedar season. The primary efficacy variable was change from baseline in 12-hour reflective TNSS over the entire 2 weeks of treatment. TNSS consisted of nasal congestion, itchy nose, runny nose, and sneezing, scored on a 4-point (0 to 3) scale with a maximum possible daily score of 24.
MECHANISM OF ACTION:
ASTEPRO® WORKS DIRECTLY AT THE SITE OF NASAL ALLERGIES12
INS, intranasal steroid; SAR, seasonal allergic rhinitis; TNSS, Total Nasal Symptom Score.
*Compared with other steroid allergy sprays, on day 1.
REFERENCES:
1. Hsu S-N, Stevens DA, Sajjad F, Salapatek AM. Onset of action of azelastine HCl nasal spray 0.15% evaluated in an environmental exposure chamber. Poster presented at: ACAAI Annual Scientific Meeting; November 10-14, 2022; Louisville, KY. 2. Shah S, Berger W, Lumry W, La Force C, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray and azelastine 0.10% nasal spray in patients with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30(6):628-633. doi:10.2500/aap.2009.30.3296 3. Flonase® website. Accessed March 2, 2022. https://www.flonase.com/faqs/ 4. Flonase®. Prescribing Information. GlaxoSmithKline; 2019. 5. GSK health partner website. Accessed March 2, 2022. https://www.gskhealthpartner.com/en-us/respiratory-health/brands/flonase-products/flonase/dosing-administration-flonase-allergy-relief/ 6. Nasacort® website. Accessed March 2, 2022. https://www.nasacort.com/allergy-nasal-spray-nasacort-faqs/ 7. Nasacort® AQ. Prescribing information. Sanofi-aventis U.S. LLC; 2008. 8. Rhinocort website. Accessed November 11, 2021. https://www.rhinocort.com/allergy-nasal-spray/adult-otc-spray 9. Data on file. Bayer. MP440 CSR. Symptom scores. 10. van Bavel J, Howland WC, Amar NJ, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray administered once daily in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2009;30(5):512-518. doi:10.2500/aap.2009.30.3284 11. Howland WC, Amar NJ, Wheeler W, Sacks H. Efficacy and safety of azelastine 0.15% nasal spray administered once daily in patients with allergy to Texas mountain cedar pollen. Int Forum Allergy Rhinol. 2011;1(4):275-279. doi:10.1002/alr.20065 12. Horak F. Effectiveness of twice daily azelastine nasal spray in patients with seasonal allergic rhinitis. Ther Clin Risk Management. 2008;4(5):1009-1022. 13. NDA 213872. 14. Astepro®. Prescribing information. Meda Pharmaceuticals Inc; 2018. 15. US Department of Health and Human Services. Pediatric Postmarketing Pharmacovigilance and Drug Utilization Review. November 3, 2016. Accessed October 28, 2021. https://www.fda.gov/media/100100/download 16. Astelin. Prescribing information. Meda Pharmaceuticals Inc; 2014.